Замещенный триптамин


Замещенные триптамины , или просто триптамины , также известные как аналоги серотонина (т. е. аналоги 5-гидрокситриптамина ), представляют собой органические соединения , которые можно считать производными от самого триптамина . Молекулярные структуры всех триптаминов содержат индольное кольцо, соединенное с аминогруппой (NH 2 ) через этиловую (−CH2–CH2−) боковую цепь . В замещенных триптаминах индольное кольцо, боковая цепь и/или аминогруппа модифицированы путем замены одного из атомов водорода (H) другой группой.
Известные триптамины включают серотонин , важный нейромедиатор , и мелатонин , гормон, участвующий в регуляции цикла сна и бодрствования. Триптаминовые алкалоиды встречаются в грибах , растениях и животных ; и иногда используются людьми для неврологического или психотропного воздействия вещества. Известные примеры триптаминовых алкалоидов включают псилоцибин (от « псилоцибиновых грибов ») и ДМТ . В Южной Америке диметилтриптамин получают из многочисленных растительных источников, таких как чакруна , и его часто используют в отварах аяуаски . Также было изготовлено много синтетических триптаминов, включая лекарство от мигрени суматриптан и психоделические препараты . Исследование 2022 года показало, что разнообразие триптаминов, присутствующих в лесных грибах, может влиять на терапевтическое воздействие. [1]
Структура триптамина, в частности его индольное кольцо, может быть частью структуры некоторых более сложных соединений, например: ЛСД , ибогаин , митрагинин и йохимбин . Тщательное исследование десятков соединений триптамина было опубликовано Энн и Александром Шульгиным под названием TiHKAL .
Список замещенных триптаминов
This list is incomplete; you can help by adding missing items. (October 2021) |
| Химическая структура | Краткое имя | Источник | Замена кольца | Р Н1 | Р Н2 | Полное имя | Номер CAS |
|---|---|---|---|---|---|---|---|
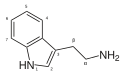 | Триптамин | Животные, растения, грибы | ЧАС | ЧАС | ЧАС | 3-(2-аминоэтил)индол / 2-(1H-индол-3-ил)этанамин | 61-54-1 |
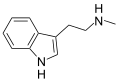 | НМТ | Растения | ЧАС | ЧАС | Гл 3 | N-methyltryptamine | 61-49-4 |
 | 2-HO-NMT | Plants | 2-OH | H | CH3 | 2-hydroxy-N-methyltryptamine | 106987-89-7 |
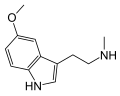 | 5-MeO-NMT | Plants | 5-OCH3 | H | CH3 | 5-methoxy-N-methyltryptamine | 2009-03-2 |
 | Serotonin | Animals, plants | 5-OH | H | H | 5-hydroxytryptamine | 50-67-9 |
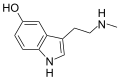 | Nω-Methylserotonin (norbufotenin) | Plants | 5-OH | H | CH3 | 5-hydroxy-N-methyltryptamine | 1134-01-6 |
 | Bufotenin | Animals, plants, fungi | 5-OH | CH3 | CH3 | 5-hydroxy-N,N-dimethyltryptamine | 487-93-4 |
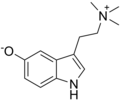 | Bufotenidine | Amphibians | 5-O− | (CH3)3 | 3-[2-(trimethylazaniumyl)ethyl]-1H-indol-5-olate | 487-91-2 | |
 | Melatonin | Animals, plants, microbes | 5-OCH3 | H | O=C-CH3 | 5-methoxy-N-acetyltryptamine | 73-31-4 |
 | N-Acetylserotonin | Animals | 5-OH | H | O=C-CH3 | 5-hydroxy-N-acetyltryptamine | 1210-83-9 |
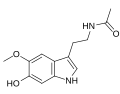 | 6-Hydroxymelatonin | Animals | 5-OCH3, 6-OH | H | O=C-CH3 | N-[2-(6-Hydroxy-5-methoxy-1H-indol-3-yl)ethyl]acetamide | 2208-41-5 |
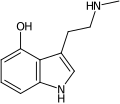 | 4-HO-NMT | Fungi | 4-OH | H | CH3 | 4-hydroxy-N-methyltryptamine | 28363-70-4 |
 | Psilocin | Fungi | 4-OH | CH3 | CH3 | 4-hydroxy-N,N-dimethyltryptamine | 520-53-6 |
 | Norbaeocystin | Fungi | 4-OPO3H2 | H | H | 4-phosphoryloxy-tryptamine | 21420-59-7 |
 | Baeocystin | Fungi | 4-OPO3H2 | H | CH3 | 4-phosphoryloxy-N-methyl-tryptamine | 21420-58-6 |
 | Psilocybin | Fungi | 4-OPO3H2 | CH3 | CH3 | 4-phosphoryloxy-N,N-dimethyltryptamine | 520-52-5 |
 | Aeruginascin | Fungi | 4-OPO3H2 | (CH3)3 | [3-[2-(trimethylazaniumyl)ethyl]-1H-indol-4-yl] hydrogen phosphate | 114264-95-8 | |
 | DMT | Animals, plants | H | CH3 | CH3 | N,N-dimethyltryptamine | 61-50-7 |
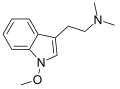 | Lespedamine | Plants | 1-OCH3 | CH3 | CH3 | 1-methoxy-N,N-dimethyltryptamine | 4335-93-7 |
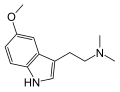 | 5-MeO-DMT | Animals, plants | 5-OCH3 | CH3 | CH3 | 5-methoxy-N,N-dimethyltryptamine | 1019-45-0 |
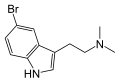 | 5-Bromo-DMT | Marine sponges, invertebrates | 5-Br | CH3 | CH3 | 5-bromo-N,N-dimethyltryptamine | 17274-65-6 |
 | 6-Bromotryptamine | Marine invertebrates | 6-Br | H | H | 6-bromotryptamine | 96624-18-9 |
 | 5,6-Dibromotryptamine | Marine invertebrates | 5,6-Br | H | H | 5,6-dibromotryptamine | |
 | 5,6-Dibromo-NMT | Marine invertebrates | 5,6-Br | H | CH3 | 5,6-dibromo-N-methyltryptamine | |
 | 5,6-Dibromo-DMT | Marine sponges, invertebrates | 5,6-Br | CH3 | CH3 | 5,6-dibromo-N,N-dimethyltryptamine | 72853-80-6 |
 | Desformylflustrabromine | Marine invertebrates | 2-(α,α-dimethylallyl), 6-Br | H | CH3 | 2-[6-bromo-2-(2-methylbut-3-en-2-yl)-1H-indol-3-yl]-N-methylethanamine | 474657-72-2 |
 | Convolutindole A | Marine invertebrates | 2,4,6-Br, 1,7-OCH3 | CH3 | CH3 | 1,7-dimethoxy-2,4,6-tribromo-N,N-dimethyltryptamine | 443356-86-3 |
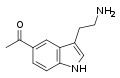 | Acetryptine | artificial | 5-COCH3 | H | H | 5-Acetyltryptamine | 3551-18-6 |
 | 5-BT | artificial | 5-OCH2C6H5 | H | H | 5-Benzyloxytryptamine | 20776-45-8 |
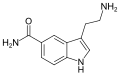 | 5-CT | artificial | 5-CONH2 | H | H | 5-Carboxamidotryptamine | 74885-09-9 |
 | 5-(Nonyloxy)tryptamine | artificial | 5-O(CH2)8CH3 | H | H | 5-nonyloxytryptamine | 157798-12-4 |
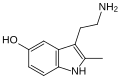 | 2-Methyl-5-hydroxytryptamine | artificial | 2-CH3, 5-OH | H | H | 3-(2-aminoethyl)-2-methyl-1H-indol-5-ol | 78263-90-8 |
 | NET | artificial | H | H | CH2CH3 | N-ethyltryptamine | 61-53-0 |
 | NiPT | artificial | H | H | CH(CH3)2 | N-isopropyltryptamine | 14121-10-9 |
 | NcPT | artificial | H | H | C3H5 | N-cyclopropyltryptamine | |
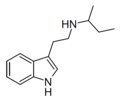 | NSBT | artificial | H | H | CH(CH3)CH2CH3 | N-sec-butyltryptamine | |
 | NTBT | artificial | H | H | C(CH3)3 | N-[2-(1H-indol-3-yl)ethyl]-2-methylpropan-2-amine | |
 | 5-MeO-T-NBOMe | artificial | 5-OCH3 | H | CH2C6H4(o-OCH3) | 5-methoxy-N-(ortho-methoxybenzyl)tryptamine | 1335331-37-7 |
 | 5-MT-NB3OMe [2] | artificial | 5-OCH3 | H | CH2C6H4(m-OCH3) | 5-methoxy-N-(meta-methoxybenzyl)tryptamine | 1648553-42-7 |
 | 5-MeO-NBpBrT | artificial | 5-OCH3 | H | CH2C6H4(p-Br) | N-(4-Bromobenzyl)-2-(5-methoxy-1H-indol-3-yl)ethanamine | 155639-13-7 |
 | 5-MeO-34MPEMT [3] | artificial | 5-OCH3 | CH3 | CH2CH2C6H3(p,m-OCH3) | N-methyl-N-[2-(3,4-dimethoxyphenyl)ethyl]-2-(5-methoxy-1H-indol-3-yl)ethanamine | |
 | Idalopirdine | artificial | 6-F | H | CH2C6H4(m-OCH2CF2CF2H) | 2-(6-Fluoro-1H-indol-3-yl)-N-(3-(2,2,3,3-tetrafluoropropoxy)benzyl)ethanamine | 467459-31-0 |
 | Z2876442907[4] | artificial | 4-CH3 | H | CH2(C3HNS)COOCH2CH3 | ethyl 2-({[2-(4-methyl-1H-indol-3-yl)ethyl]amino}methyl)-1,3-thiazole-5-carboxylate | |
 | Pyr-T | artificial | H | (CH2)4 | 3-[2-(Pyrrolidin-1-yl)ethyl]-1H-indole | 14008-96-9 | |
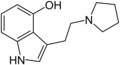 | 4-HO-pyr-T | artificial | 4-OH | (CH2)4 | 3-[2-(Pyrrolidin-1-yl)ethyl]-1H-indol-4-ol | 63097-26-7 | |
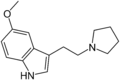 | 5-MeO-pyr-T | artificial | 5-OCH3 | (CH2)4 | 5-Methoxy-3-[2-(pyrrolidin-1-yl)ethyl]-1H-indole | 3949-14-2 | |
 | Indolylethylfentanyl | artificial | H | (CH2)5-4-N(COCH2CH3)C6H5 | N-[1-[2-(1H-indol-3-yl)ethyl]piperidin-4-yl]-N-phenylpropanamide | 58399-46-5 | |
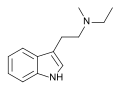 | MET | artificial | H | CH3 | CH2CH3 | N-Methyl-N-ethyltryptamine | 5599-69-9 |
 | MPT | artificial | H | CH3 | CH2CH2CH3 | N-Methyl-N-propyltryptamine | 850032-72-3 |
 | MiPT | artificial | H | CH3 | CH(CH3)2 | N-Methyl-N-isopropyltryptamine | 96096-52-5 |
 | McPT | artificial | H | CH3 | C3H5 | N-Methyl-N-cyclopropyltryptamine | 1373918-63-8 |
 | EcPT | artificial | H | CH2CH3 | C3H5 | N-ethyl-N-cyclopropyltryptamine | |
 | PcPT | artificial | H | CH2CH2CH3 | C3H5 | N-propyl-N-cyclopropyltryptamine | |
 | iPcPT | artificial | H | CH(CH3)2 | C3H5 | N-isopropyl-N-cyclopropyltryptamine | |
 | DcPT | artificial | H | C3H5 | C3H5 | N,N-dicyclopropyltryptamine | 1373918-62-7 |
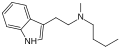 | MBT | artificial | H | CH3 | (CH2)3CH3 | N-Methyl-N-butyltryptamine | 848130-12-1 |
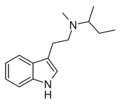 | MSBT | artificial | H | CH3 | CH(CH3)CH2CH3 | N-Methyl-N-sec-butyltryptamine | |
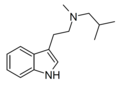 | MiBT | artificial | H | CH3 | CH2CH(CH3)2 | N-Methyl-N-iso-butyltryptamine | |
 | McPMT | artificial | H | CH3 | CH2C3H5 | N-Methyl-N-(cyclopropylmethyl)tryptamine | |
 | DET | artificial | H | CH2CH3 | CH2CH3 | N,N-diethyltryptamine | 61-51-8 |
 | EPT | artificial | H | CH2CH3 | CH2CH2CH3 | N-Ethyl-N-propyltryptamine | 850032-68-7 |
 | EiPT | artificial | H | CH2CH3 | CH(CH3)2 | N-Ethyl-N-isopropyltryptamine | 848130-11-0 |
 | DPT | artificial | H | CH2CH2CH3 | CH2CH2CH3 | N,N-dipropyltryptamine | 61-52-9 |
 | PiPT | artificial | H | CH2CH2CH3 | CH(CH3)2 | N-Propyl-N-isopropyltryptamine | 1354632-00-0 |
 | DiPT | artificial | H | CH(CH3)2 | CH(CH3)2 | N,N-diisopropyltryptamine | 14780-24-6 |
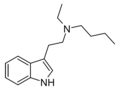 | EBT | artificial | H | CH2CH3 | (CH2)3CH3 | N-ethyl-N-butyltryptamine | |
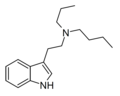 | PBT | artificial | H | CH2CH2CH3 | (CH2)3CH3 | N-propyl-N-butyltryptamine | |
 | iPsBT | artificial | H | CH(CH3)2 | CH(CH3)CH2CH3 | N-isopropyl-N-sec-butyltryptamine | |
 | DBT | artificial | H | (CH2)3CH3 | (CH2)3CH3 | N,N-dibutyltryptamine | 15741-77-2 |
 | DIBT | artificial | H | CH2CH(CH3)2 | CH2CH(CH3)2 | N,N-diisobutyltryptamine | 63938-64-7 |
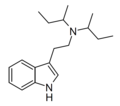 | DSBT | artificial | H | CH(CH3)CH2CH3 | CH(CH3)CH2CH3 | N,N-disecbutyltryptamine | |
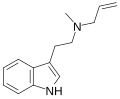 | MALT | artificial | H | CH3 | H2C=CH-CH2 | N-methyl-N-allyltryptamine | 1366416-29-6 |
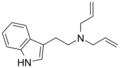 | DALT | artificial | H | H2C=CH-CH2 | H2C=CH-CH2 | N,N-diallyltryptamine | 60676-77-9 |
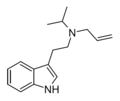 | ALiPT | artificial | H | H2C=CH-CH2 | CH(CH3)2 | N-allyl-N-isopropyltryptamine | |
 | 2-Methyl-DMT | artificial | 2-CH3 | CH3 | CH3 | (2-(2-methyl-1H-indol-3-yl)-1-methyl-ethyl)dimethylamine | 1080-95-1 |
 | 2-Me-DET | artificial | 2-CH3 | CH2CH3 | CH2CH3 | N,N-Diethyl-2-(2-methyl-1H-indol-3-yl)ethan-1-amine | 26628-88-6 |
 | 4-Amino-DMT [5] | artificial | 4-NH2 | CH3 | CH3 | 4-amino-N,N-dimethyltryptamine | 60331-61-5 |
 | 4-Methyl-DMT | artificial | 4-CH3 | CH3 | CH3 | 4,N,N-trimethyltryptamine | 28289-23-8 |
 | 4-MeO-DMT | artificial | 4-OCH3 | CH3 | CH3 | 4-methoxy-N,N-dimethyltryptamine | 3965-97-7 |
 | 4-MeO-MiPT | artificial | 4-OCH3 | CH3 | CH(CH3)2 | 4-methoxy-N-methyl-N-isopropyltryptamine | 96096-53-6 |
 | 4-MeO-DiPT | artificial | 4-OCH3 | CH(CH3)2 | CH(CH3)2 | 4-methoxy-N,N-diisopropyltryptamine | |
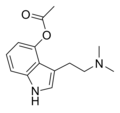 | 4-AcO-DMT | artificial | 4-OCOCH3 | CH3 | CH3 | 4-acetoxy-N,N-dimethyltryptamine | 92292-84-7 |
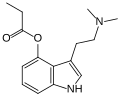 | 4-PrO-DMT | artificial | 4-OCOCH2CH3 | CH3 | CH3 | 4-propionyloxy-N,N-dimethyltryptamine | 1373882-11-1 |
 | 4-HO-MET | artificial | 4-OH | CH3 | CH2CH3 | 4-hydroxy-N-methyl-N-ethyltryptamine | 77872-41-4 |
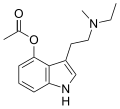 | 4-Acetoxy-MET | artificial | 4-OCOCH3 | CH3 | CH2CH3 | 4-acetoxy-N-methyl-N-ethyltryptamine | 1445751-40-5 |
 | 4-PO-MET | artificial | 4-OPO3H2 | CH3 | CH2CH3 | 4-phosphoryloxy-N-methyl-N-ethyltryptamine | |
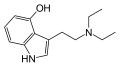 | 4-HO-DET | artificial | 4-OH | CH2CH3 | CH2CH3 | 4-hydroxy-N,N-diethyltryptamine | 22204-89-3 |
 | 4-Acetoxy-DET | artificial | 4-OCOCH3 | CH2CH3 | CH2CH3 | 4-acetoxy-N,N-diethyltryptamine | 1135424-15-5 |
 | 4-PO-DET | artificial | 4-OPO3H2 | CH2CH3 | CH2CH3 | 4-phosphoryloxy-N,N-diethyltryptamine | 60480-02-6 |
 | 4-HO-EPT | artificial | 4-OH | CH2CH3 | CH2CH2CH3 | 4-hydroxy-N-ethyl-N-propyltryptamine | 2595431-59-5 |
 | 4-PO-EPT | artificial | 4-OPO3H2 | CH2CH3 | CH2CH2CH3 | 4-phosphoryloxy-N-ethyl-N-propyltryptamine | |
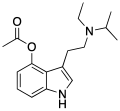 | 4-AcO-EiPT | artificial | 4-OCOCH3 | CH2CH3 | CH(CH3)2 | 4-acetoxy-N-ethyl-N-isopropyltryptamine | |
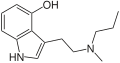 | 4-HO-MPT | artificial | 4-OH | CH3 | CH2CH2CH3 | 4-hydroxy-N-methyl-N-propyltryptamine | 763035-03-6 |
 | 4-HO-MiPT | artificial | 4-OH | CH(CH3)2 | CH3 | 4-hydroxy-N-isopropyl-N-methyltryptamine | 77872-43-6 |
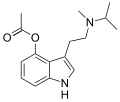 | 4-Acetoxy-MiPT | artificial | 4-OCOCH3 | CH3 | CH(CH3)2 | 4-acetoxy-N-methyl-N-isopropyltryptamine | 1024612-25-6 |
 | 4-HO-MALT[6] | artificial | 4-OH | CH3 | H2C=CH-CH2 | 4-hydroxy-N-Methyl-N-allyltryptamine | |
 | 4-AcO-MALT[7] | artificial | 4-OCOCH3 | CH3 | H2C=CH-CH2 | 4-acetoxy-N-Methyl-N-allyltryptamine | |
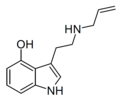 | 4-HO-NALT[8] | artificial | 4-OH | H | H2C=CH-CH2 | 4-hydroxy-N-allyltryptamine | |
 | 4-HO-MSBT | artificial | 4-OH | CH(CH3)CH2CH3 | CH3 | 4-hydroxy-N-sec-butyl-N-methyltryptamine | |
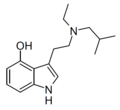 | 4-HO-EiBT | artificial[9] | 4-OH | CH2CH(CH3)2 | CH2CH3 | 4-hydroxy-N-iso-butyl-N-ethyltryptamine | |
 | 4-HO-McPT | artificial | 4-OH | C3H5 | CH3 | 4-hydroxy-N-cyclopropyl-N-methyltryptamine | 2883663-05-4 |
 | 4-HO-McPeT | artificial | 4-OH | C5H9 | CH3 | 4-hydroxy-N-cyclopentyl-N-methyltryptamine | 77872-48-1 |
 | 4-HO-McPMT [10] | artificial | 4-OH | CH2C3H5 | CH3 | 4-hydroxy-N-cyclopropylmethyl-N-methyltryptamine | |
 | 4-HO-DPT | artificial | 4-OH | CH2CH2CH3 | CH2CH2CH3 | 4-hydroxy-N,N-dipropyltryptamine | 63065-88-3 |
 | 4-AcO-DPT | artificial | 4-OCOCH3 | CH2CH2CH3 | CH2CH2CH3 | 4-acetoxy-N,N-dipropyltryptamine | 1445751-75-6 |
 | 4-HO-PiPT | artificial | 4-OH | CH2CH2CH3 | CH(CH3)2 | 4-hydroxy-N-propyl-N-isopropyltryptamine | |
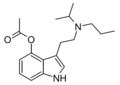 | 4-AcO-PiPT | artificial | 4-OCOCH3 | CH2CH2CH3 | CH(CH3)2 | 4-acetoxy-N-propyl-N-isopropyltryptamine | |
 | 4-HO-DIPT | artificial | 4-OH | CH(CH3)2 | CH(CH3)2 | 4-hydroxy-N,N-diisopropyltryptamine | 132328-45-1 |
 | 4-Acetoxy-DiPT | artificial | 4-OCOCH3 | CH(CH3)2 | CH(CH3)2 | 4-acetoxy-N,N-diisopropyltryptamine | 936015-60-0 |
 | 4-PrO-DiPT | artificial | 4-OCOCH2CH3 | CH(CH3)2 | CH(CH3)2 | 4-propionyloxy-N,N-diisopropyltryptamine | 1373882-13-3 |
 | FT-104 | artificial | 4-OCO(CH2)3COOH | CH(CH3)2 | CH(CH3)2 | 4-glutaryloxy-N,N-diisopropyltryptamine | |
 | 4-PO-DiPT | artificial | 4-OPO3H2 | CH(CH3)2 | CH(CH3)2 | 4-phosphoryloxy-N,N-diisopropyltryptamine | 1373882-09-7 |
 | 4-HO-DALT | artificial | 4-OH | H2C=CH-CH2 | H2C=CH-CH2 | 4-hydroxy-N,N-diallyltryptamine | |
 | 4-AcO-DALT | artificial | 4-OCOCH3 | H2C=CH-CH2 | H2C=CH-CH2 | 4-acetoxy-N,N-diallyltryptamine | 1445751-71-2 |
 | 4-HO-DBT | artificial | 4-OH | (CH2)3CH3 | (CH2)3CH3 | 4-hydroxy-N,N-dibutyltryptamine | 63065-89-4 |
 | 4-HO-DIBT | artificial | 4-OH | CH2CH(CH3)2 | CH2CH(CH3)2 | 4-hydroxy-N,N-diisobutyltryptamine | |
 | 4-HO-DSBT | artificial | 4-OH | CH(CH3)CH2CH3 | CH(CH3)CH2CH3 | 4-hydroxy-N,N-disecbutyltryptamine | 127507-01-1 |
 | 5-MeO-MET | artificial | 5-OCH3 | CH2CH3 | CH3 | 5-methoxy-N-Methyl-N-ethyltryptamine | 16977-53-0 |
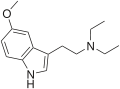 | 5-MeO-DET | artificial | 5-OCH3 | CH2CH3 | CH2CH3 | 5-methoxy-N,N-diethyltryptamine | 2454-70-8 |
 | 5-MeO-MPT | artificial | 5-OCH3 | CH3 | CH2CH2CH3 | 5-methoxy-N-methyl-N-propyltryptamine | |
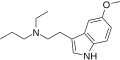 | 5-MeO-EPT | artificial | 5-OCH3 | CH2CH3 | CH2CH2CH3 | 5-methoxy-N-ethyl-N-propyltryptamine | 850032-67-6 |
 | 5-MeO-DPT | artificial | 5-OCH3 | CH2CH2CH3 | CH2CH2CH3 | 5-methoxy-N,N-dipropyltryptamine | 69496-75-9 |
 | 5-MeO-MALT | artificial | 5-OCH3 | H2C=CH-CH2 | CH3 | 5-methoxy-N-Methyl-N-allyltryptamine | 1373918-64-9 |
 | 5-MeO-DALT | artificial | 5-OCH3 | H2C=CH-CH2 | H2C=CH-CH2 | 5-methoxy-N,N-diallyltryptamine | 928822-98-4 |
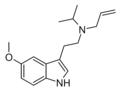 | 5-MeO-ALiPT | artificial | 5-OCH3 | H2C=CH-CH2 | CH2CH(CH3)2 | 5-methoxy-N-allyl-N-isopropyltryptamine | |
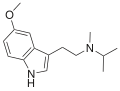 | 5-MeO-MiPT | artificial | 5-OCH3 | CH3 | CH(CH3)2 | 5-methoxy-N,N-methylisopropyltryptamine | 96096-55-8 |
 | 5,6-MeO-MiPT | artificial | 5-OCH3, 6-OCH3 | CH3 | CH(CH3)2 | 5,6-dimethoxy-N,N-methylisopropyltryptamine | |
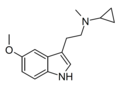 | 5-MeO-McPT | artificial | 5-OCH3 | CH3 | C3H5 | 5-methoxy-N-methyl-N-cyclopropyltryptamine | |
 | 5-MeO-McBT | artificial | 5-OCH3 | CH3 | C4H7 | 5-methoxy-N-methyl-N-cyclobutyltryptamine | |
 | 5-MeO-EiPT | artificial | 5-OCH3 | CH2CH3 | CH(CH3)2 | 5-methoxy-N-ethyl-N-isopropyltryptamine | 850032-66-5 |
 | 5-MeO-PiPT | artificial | 5-OCH3 | CH2CH2CH3 | CH(CH3)2 | 5-methoxy-N-propyl-N-isopropyltryptamine | |
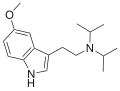 | 5-MeO-DIPT | artificial | 5-OCH3 | CH(CH3)2 | CH(CH3)2 | 5-methoxy-N,N-diisopropyltryptamine | 4021-34-5 |
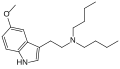 | 5-MeO-DBT | artificial | 5-OCH3 | (CH2)3CH3 | (CH2)3CH3 | 5-methoxy-N,N-dibutyltryptamine | 73785-42-9 |
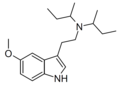 | 5-MeO-DSBT | artificial | 5-OCH3 | CH(CH3)CH2CH3 | CH(CH3)CH2CH3 | 5-methoxy-N,N-di-sec-butyltryptamine | |
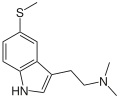 | 5-MeS-DMT | artificial | 5-SCH3 | CH3 | CH3 | 5-methylthio-N,N-dimethyltryptamine | 5102-11-4 |
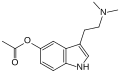 | 5-AcO-DMT | artificial | 5-OCOCH3 | CH3 | CH3 | 5-acetoxy-N,N-dimethyltryptamine | 16977-50-7 |
 | 5-AcO-MET [11] | artificial | 5-OCOCH3 | CH3 | CH2CH3 | 5-acetoxy-N-methyl-N-ethyltryptamine | |
 | 5-AcO-DET | artificial | 5-OCOCH3 | CH2CH3 | CH2CH3 | 5-acetoxy-N,N-diethyltryptamine | |
 | 5-AcO-EPT [12] | artificial | 5-OCOCH3 | CH2CH3 | CH2CH2CH3 | 5-acetoxy-N-ethyl-N-propyltryptamine | |
 | 5-AcO-DPT | artificial | 5-OCOCH3 | CH2CH2CH3 | CH2CH2CH3 | 5-acetoxy-N,N-dipropyltryptamine | |
 | 5-AcO-MiPT | artificial | 5-OCOCH3 | CH3 | CH(CH3)2 | 5-acetoxy-N-methyl-N-isopropyltryptamine | |
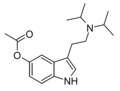 | 5-AcO-DiPT | artificial | 5-OCOCH3 | CH(CH3)2 | CH(CH3)2 | 5-acetoxy-N,N-diisopropyltryptamine | |
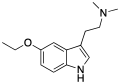 | 5-Ethoxy-DMT | artificial | 5-OCH2CH3 | CH3 | CH3 | 5-ethoxy-N,N-dimethyltryptamine | 855245-09-9 |
 | 5-Ethoxy-MET | artificial | 5-OCH2CH3 | CH3 | CH2CH3 | 5-ethoxy-N-methyl-N-ethyltryptamine | |
 | 5-Ethoxy-DET | artificial | 5-OCH2CH3 | CH2CH3 | CH2CH3 | 5-ethoxy-N,N-diethyltryptamine | |
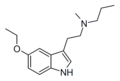 | 5-Ethoxy-MPT | artificial | 5-OCH2CH3 | CH3 | CH2CH2CH3 | 5-ethoxy-N-methyl-N-propyltryptamine | |
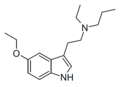 | 5-Ethoxy-EPT | artificial | 5-OCH2CH3 | CH2CH3 | CH2CH2CH3 | 5-ethoxy-N-ethyl-N-propyltryptamine | |
 | 5-Ethoxy-DPT | artificial | 5-OCH2CH3 | CH2CH2CH3 | CH2CH3 | 5-ethoxy-N,N-dipropyltryptamine | |
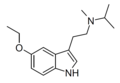 | 5-Ethoxy-MiPT | artificial | 5-OCH2CH3 | CH3 | CH(CH3)2 | 5-ethoxy-N-methyl-N-isopropyltryptamine | |
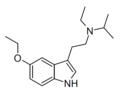 | 5-Ethoxy-EiPT | artificial | 5-OCH2CH3 | CH2CH3 | CH(CH3)2 | 5-ethoxy-N-ethyl-N-isopropyltryptamine | |
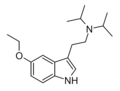 | 5-Ethoxy-DiPT | artificial | 5-OCH2CH3 | CH(CH3)2 | CH(CH3)2 | 5-ethoxy-N,N-diisopropyltryptamine | |
 | 5-Ethoxy-DALT | artificial | 5-OCH2CH3 | H2C=CH-CH2 | H2C=CH-CH2 | 5-ethoxy-N,N-diallyltryptamine | |
 | 5-BnO-DMT | artificial | 5-OCH2C6H5 | CH3 | CH3 | 5-benzyloxy-N,N-dimethyltryptamine | 101832-88-6 |
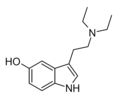 | 5-HO-DET | artificial | 5-OH | CH2CH3 | CH2CH3 | 5-hydroxy-N,N-diethyltryptamine | 14009-42-8 |
 | 5-HO-DPT | artificial | 5-OH | CH2CH2CH3 | CH2CH2CH3 | 5-hydroxy-N,N-dipropyltryptamine | 36288-75-2 |
 | 5-HO-MiPT | artificial | 5-OH | CH3 | CH(CH3)2 | 5-hydroxy-N-methyl-N-isopropyltryptamine | |
 | 5-HO-DiPT | artificial | 5-OH | CH(CH3)2 | CH(CH3)2 | 5-hydroxy-N,N-diisopropyltryptamine | 36288-76-3 |
 | 5-Methyl-DMT (5,N,N-TMT) | artificial | 5-CH3 | CH3 | CH3 | 5,N,N-trimethyltryptamine | 22120-39-4 |
 | 5-Ethyl-DMT | artificial | 5-CH2CH3 | CH3 | CH3 | 5-ethyl-N,N-dimethyltryptamine | 171783-25-8 |
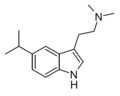 | 5-Isopropyl-DMT | artificial | 5-CH(CH3)2 | CH3 | CH3 | 5-isopropyl-N,N-dimethyltryptamine | 156281-04-8 |
 | 5-(t-Butyl)-DMT [13] | artificial | 5-C(CH3)3 | CH3 | CH3 | 5-(tert-butyl)-N,N-dimethyltryptamine | |
 | 5-Fluoro-DMT | artificial | 5-F | CH3 | CH3 | 5-fluoro-N,N-dimethyltryptamine | 22120-36-1 |
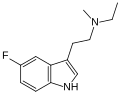 | 5-Fluoro-MET | artificial | 5-F | CH3 | CH2CH3 | 5-fluoro-N-methyl-N-ethyltryptamine | |
 | 5-Fluoro-DET | artificial | 5-F | CH2CH3 | CH2CH3 | 5-fluoro-N,N-diethyltryptamine | |
 | 5-Fluoro-EPT | artificial | 5-F | CH2CH3 | CH2CH2CH3 | 5-fluoro-N-ethyl-N-propyltryptamine | |
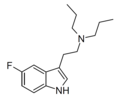 | 5-Fluoro-DPT | artificial | 5-F | CH2CH2CH3 | CH2CH2CH3 | 5-fluoro-N,N-dipropyltryptamine | |
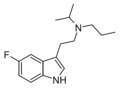 | 5-Fluoro-PiPT | artificial | 5-F | CH2CH2CH3 | CH(CH3)2 | 5-fluoro-N-propyl-N-isopropyltryptamine | |
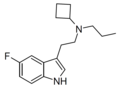 | 5-Fluoro-PcBT | artificial | 5-F | CH2CH2CH3 | CH(CH2)3 | 5-fluoro-N-propyl-N-cyclobutyltryptamine | |
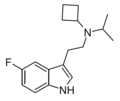 | 5-Fluoro-iPcBT | artificial | 5-F | CH(CH3)2 | CH(CH2)3 | 5-fluoro-N-isopropyl-N-cyclobutyltryptamine | |
 | 5-Fluoro-DiPT | artificial | 5-F | CH(CH3)2 | CH(CH3)2 | 5-fluoro-N,N-diisoproptryptamine | |
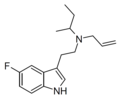 | 5-Fluoro-sBALT | artificial | 5-F | CH(CH3)CH2CH3 | CH2CH=CH2 | 5-fluoro-N-sec-butyl-N-allyltryptamine | |
 | 5-Fluoro-M1MALT | artificial | 5-F | CH3 | CH(CH3)CH=CH2 | 5-fluoro-N-methyl-N-(1-methylallyl)tryptamine | |
 | 5-Chloro-DMT | artificial | 5-Cl | CH3 | CH3 | 5-chloro-N,N-dimethyltryptamine | 22120-32-7 |
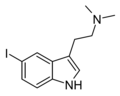 | 5-Iodo-DMT | artificial | 5-I | CH3 | CH3 | 5-iodo-N,N-dimethyltryptamine | 22120-38-3 |
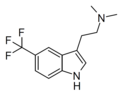 | 5-TFM-DMT | artificial | 5-CF3 | CH3 | CH3 | 5-(trifluoromethyl)-N,N-dimethyltryptamine | 2418713-32-1 |
 | 5-TFMO-DMT[14] | artificial | 5-OCF3 | CH3 | CH3 | 5-(trifluoromethoxy)-N,N-dimethyltryptamine | |
 | 5-Nitro-DMT[15] | artificial | 5-NO2 | CH3 | CH3 | 5-nitro-N,N-dimethyltryptamine | 69937-13-9 |
 | 5-CN-DMT | artificial | 5-C≡N | CH3 | CH3 | 5-cyano-N,N-dimethyltryptamine | 17380-42-6 |
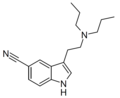 | 5-CN-DPT | artificial | 5-C≡N | CH2CH2CH3 | CH2CH2CH3 | 5-cyano-N,N-dipropyltryptamine | 74885-19-1 |
 | Almotriptan | artificial | 5-(CH2SO2N(CH2)4) | CH3 | CH3 | N,N-dimethyl-2- [5-(pyrrolidin-1-ylsulfonylmethyl)- 1H-indol-3-yl]-ethanamine | 154323-57-6 |
 | Rizatriptan | artificial | 5-(CH2(N3(CH)2)) | CH3 | CH3 | N,N-dimethyl-2-[5-(1H-1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethanamine | 145202-66-0 |
 | Sumatriptan | artificial | 5-(CH2SO2NHCH3) | CH3 | CH3 | 1-[3-(2-Dimethylaminoethyl)-1H-indol-5-yl]-N-methyl-methanesulfonamide | 103628-46-2 |
 | Zolmitriptan | artificial | 5-(CHNHC=OOCH2) | CH3 | CH3 | 5-( 4-(S)-1,3-oxazolidin-2-one)-N,N-dimethyltryptamine | 139264-17-8 |
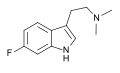 | 6-Fluoro-DMT | artificial | 6-F | CH3 | CH3 | 6-fluoro-N,N-dimethyltryptamine | 1511-31-5 |
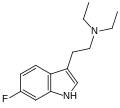 | 6-Fluoro-DET[16] | artificial | 6-F | CH2CH3 | CH2CH3 | 6-fluoro-N,N-diethyltryptamine | 2836-69-3 |
 | 6-Chloro-DMT | artificial | 6-Cl | CH3 | CH3 | 6-chloro-N,N-dimethyltryptamine | 25390-72-1 |
 | 6-Methyl-DMT | artificial | 6-CH3 | CH3 | CH3 | 6,N,N-trimethyltryptamine | |
 | 6-Hydroxy-DMT | artificial | 6-OH | CH3 | CH3 | 6-hydroxy-N,N-dimethyltryptamine | 1476-33-1 |
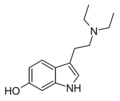 | 6-Hydroxy-DET | artificial | 6-OH | CH3 | CH3 | 6-hydroxy-N,N-diethyltryptamine | 1476-59-1 |
 | 6-Methoxy-DMT | artificial | 6-OCH3 | CH3 | CH3 | 6-methoxy-N,N-dimethyltryptamine | 2426-88-2 |
 | 7-Methyl-DMT | artificial | 7-CH3 | CH3 | CH3 | 7,N,N-trimethyltryptamine | 65882-39-5 |
 | 7-Ethyl-DMT | artificial | 7-CH2CH3 | CH3 | CH3 | 7-ethyl-N,N-dimethyltryptamine | |
 | 7-Chloro-DMT | artificial | 7-Cl | CH3 | CH3 | 7-chloro-N,N-dimethyltryptamine | |
 | 7-Bromo-DMT[17] | artificial | 7-Br | CH3 | CH3 | 7-bromo-N,N-dimethyltryptamine | 74798-68-8 |
 | 7-Methoxy-DMT | artificial | 7-OCH3 | CH3 | CH3 | 7-methoxy-N,N-dimethyltryptamine | |
 | 7-Methoxy-MiPT | artificial | 7-OCH3 | CH3 | CH(CH3)2 | 7-methoxy-N-methyl-N-isopropyltryptamine | |
 | 1-Methylpsilocin | artificial | 1-CH3, 4-OH | CH3 | CH3 | 1-Methyl-3-[2-(N,N-dimethylamino)ethyl]-4-hydroxyindole | 1465-16-3 |
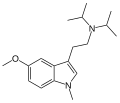 | 1-Methyl-5-MeO-DiPT | artificial | 1-CH3, 5-OCH3 | CH(CH3)2 | CH(CH3)2 | 1-methyl-5-methoxy-N,N-diisopropyltryptamine | 1373882-10-0 |
 | NBoc-DMT | artificial | 1-OCOC(CH3)3 | CH3 | CH3 | 1-(t-butoxycarbonyl)-N,N-dimethyltryptamine | |
 | NB-5-MeO-MiPT | artificial | 1-OCOC(CH3)3, 5-OCH3 | CH3 | CH(CH3)2 | 1-(t-butoxycarbonyl)-5-methoxy-N-methyl-N-isopropyltryptamine | |
 | NB-5-MeO-DALT | artificial | 1-OCOC(CH3)3, 5-OCH3 | H2C=CH-CH2 | H2C=CH-CH2 | 1-(t-butoxycarbonyl)-5-methoxy-N,N-diallyltryptamine | |
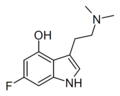 | 6-Fluoropsilocin | artificial | 4-OH,6-F | CH3 | CH3 | 4-hydroxy-6-fluoro-N,N-dimethyltryptamine | 312314-12-8 |
 | 6-Fluoro-5-MeO-DMT | artificial | 5-OCH3,6-F | CH3 | CH3 | 5-methoxy-6-fluoro-N,N-dimethyltryptamine | |
 | 5,6-Difluoro-EPT | artificial | 5-F, 6-F | CH2CH3 | CH2CH2CH3 | 5,6-difluoro-N-ethyl-N-propyltryptamine | |
 | 5-MeO-2-TMT | artificial | 2-CH3, 5-OCH3 | CH3 | CH3 | 2-(5-methoxy-2-methyl-H-indol-3-yl)-N,N-dimethylethanamine | 67292-68-6 |
 | 5-Methoxy-7,N,N-trimethyltryptamine | artificial | 5-OCH3, 7-CH3 | CH3 | CH3 | 5-Methoxy-7,N,N-trimethyltryptamine | 61018-77-7 |
 | 5-Methoxy-4,N,N-trimethyltryptamine | artificial | 4-CH3, 5-OCH3 | CH3 | CH3 | 5-Methoxy-4,N,N-trimethyltryptamine | |
 | 4-HO-5-MeO-DMT | artificial | 4-OH, 5-OCH3 | CH3 | CH3 | 4-Hydroxy-5-methoxy-N,N-dimethyltryptamine | 2433-31-0 |
 | 4-F-5-MeO-DMT | artificial | 4-F, 5-OCH3 | CH3 | CH3 | 4-Fluoro-5-Methoxy-N,N-dimethyltryptamine | 312314-18-4 |
 | 5-methoxy-7-fluoro-MET | artificial | 5-OCH3, 7-F | CH3 | CH2CH3 | 5-Methoxy-7-Fluoro-N-methyl-N-ethyltryptamine | |
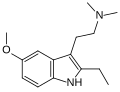 | EMDT | artificial | 2-CH2CH3, 5-OCH3 | CH3 | CH3 | 2-(2-ethyl-5-methoxy-1H-indol-3-yl)-N,N-dimethylethanamine | 263744-72-5 |
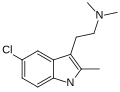 | ST-1936 | artificial | 2-CH3, 5-Cl | CH3 | CH3 | 2-(2-methyl-5-chloro-1H-indol-3-yl)-N,N-dimethylethanamine | 1210-81-7 |
 | O-4310 | artificial | 1-CH(CH3)2, 4-OH, 6-F | CH3 | CH3 | 3-[2-(dimethylamino)ethyl]-6-fluoro-1-isopropyl-1H-indol-4-ol | 885671-63-6 |
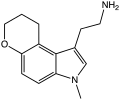 | CP-132,484 | artificial | 1-methyl-4,5-(OCH2CH2CH2) | H | H | 1-(2-aminoethyl)-3-methyl-8,9-dihydropyrano(3,2-e)indole | 143508-76-3 |
 | 4,5-DHP-DMT | artificial | 4,5-(OCH2CH2CH2) | CH3 | CH3 | 1-(2-dimethylaminoethyl)-8,9-dihydropyrano[3,2-e]indole | 135360-97-3 |
 | 4,5-DHF-DMT (P-3) | artificial[18] | 4,5-(CH2CH2O) | CH3 | CH3 | 2-(3,6-dihydro-2H-furo[2,3-e]indol-8-yl)-N,N-dimethylethan-1-amine | |
 | 4,5-Methylbenzodioxole-DMT (P-131) | artificial | 4,5-(OC(CH3)=N) | CH3 | CH3 | N,N-dimethyl-2-(2-methyl-6H-[1,3]oxazolo[4,5-e]indol-8-yl)ethan-1-amine | |
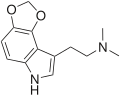 | 4,5-MDO-DMT | artificial | 4,5-(OCH2O) | CH3 | CH3 | 2-(2H,6H-[1,3]Dioxolo[4,5-e]indol-8-yl)-N,N-dimethylethan-1-amine | 81249-30-1 |
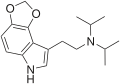 | 4,5-MDO-DiPT | artificial | 4,5-(OCH2O) | CH(CH3)2 | CH(CH3)2 | N-[2-(2H,6H-[1,3]Dioxolo[4,5-e]indol-8-yl)ethyl]-N-(propan-2-yl)propan-2-amine | 82173-82-8 |
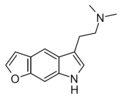 | 5,6-FUR-DMT (P-4) | artificial | 5,6-(CH=CHO) | CH3 | CH3 | 2-(7H-furo[3,2-f]indol-5-yl)-N,N-dimethylethan-1-amine | |
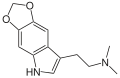 | 5,6-MDO-DMT | artificial | 5,6-(OCH2O) | CH3 | CH3 | 2-(2H,5H-[1,3]Dioxolo[4,5-f]indol-7-yl)-N,N-dimethylethan-1-amine | |
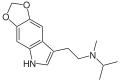 | 5,6-MDO-MiPT | artificial | 5,6-(OCH2O) | CH3 | CH(CH3)2 | N-[2-(2H,5H-[1,3]Dioxolo[4,5-f]indol-7-yl)ethyl]-N-methylpropan-2-amine | |
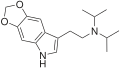 | 5,6-MDO-DiPT | artificial | 5,6-(OCH2O) | CH(CH3)2 | CH(CH3)2 | N-[2-(2H,5H-[1,3]Dioxolo[4,5-f]indol-7-yl)ethyl]-N-(propan-2-yl)propan-2-amine | |
| Chemical Structure | Short Name | Origin | Ring Substitution | RN1 | RN2 | Full Name | CAS Number |
List of substituted α-alkyltryptamines
α-Alkyltryptamines are a group of substituted tryptamines which possess an alkyl group, such as a methyl or ethyl group, attached at the alpha carbon, and in most cases no substitution on the amine nitrogen.[19][20][21] α-Alkylation of tryptamine makes it much more metabolically stable and resistant to degradation by monoamine oxidase, resulting in increased potency and greatly lengthened half-life.[21] This is analogous to α-methylation of phenethylamine into amphetamine.[21]
Many α-alkyltryptamines are drugs, acting as monoamine releasing agents, non-selective serotonin receptor agonists, and/or monoamine oxidase inhibitors,[22][23][24][25] and produce psychostimulant, entactogen, and/or psychedelic effects.[19][20][21] The most well-known of these agents are α-methyltryptamine (αMT) and α-ethyltryptamine (αET), both of which were used clinically as antidepressants for a brief period of time in the past and are abused as recreational drugs.[20][21] In accordance with its action as a dual releasing agent of serotonin and dopamine, αET has been found to produce serotonergic neurotoxicity similarly to amphetamines like MDMA and PCA, and the same is also likely to hold true for other serotonin and dopamine-releasing α-alkyltryptamines such as αMT, 5-MeO-αMT, and various others.[26]
| Structure | Common name | Chemical name | CAS number |
|---|---|---|---|
 | Tryptophan | (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid | 73-22-3 |
 | 5-Hydroxytryptophan | 2-amino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid | 4350-09-8 |
 | αMT | 1-(1H-Indol-3-yl)propan-2-amine | 299-26-3 |
 | 4-HO-αMT | 3-(2-aminopropyl)-1H-indol-4-ol | 15066-09-8 |
 | 4-Methyl-αMT | 1-methyl-2-(4-methyl-1H-indol-3-yl)-ethylamine | 3569-29-7 |
 | 5-Fluoro-αMT | 1-(5-fluoro-1H-indol-3-yl)propan-2-amine | 712-08-3 |
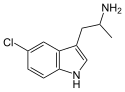 | 5-Chloro-αMT | 1-(5-Chloro-1H-indol-3-yl)propan-2-amine | 712-07-2 |
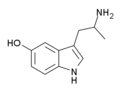 | 5-HO-αMT (αMS/α-methyl-5-HT) | 3-(2-aminopropyl)-1H-indol-5-ol | 304-52-9 |
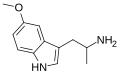 | 5-MeO-αMT | 1-(5-methoxy-1H-indol-3-yl)propan-2-amine | 1137-04-8 |
 | 5-Ethoxy-αMT | 1-(5-ethoxy-1H-indol-3-yl)propan-2-amine | 101832-83-1 |
 | 5-Isopropoxy-αMT | 1-{5-[(propan-2-yl)oxy]-1H-indol-3-yl}propan-2-amine | |
 | BW-723C86 | 1-[5-(2-Thienylmethoxy)-1H-indol-3-yl]-2-propanamine | 160521-72-2 |
 | 6-Fluoro-αMT | 1-(6-fluoro-1H-indol-3-yl)propan-2-amine | 712-11-8 |
 | 7-Chloro-AMT | 1-(7-chloro-1H-indol-3-yl)propan-2-amine | 711-99-9 |
 | AL-37350A (4,5-dihydropyrano-αMT) | (S)-(+)-1-(2-Aminopropyl)-8,9-dihydropyrano[3,2-e]indole | 362603-40-5 |
 | Compound 5 [27] | 1-(3H-benzo[e]indol-1-yl)propan-2-amine | |
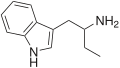 | αET | 1-(1H-indol-3-yl)butan-2-amine | 2235-90-7 |
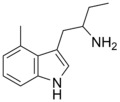 | 4-Methyl-αET | 1-(4-Methyl-1H-indol-3-yl)butan-2-amine | 28289-30-7 |
 | 4-HO-αET | 1-(4-hydroxy-1H-indol-3-yl)butan-2-amine | 28289-28-3 |
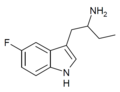 | 5-Fluoro-αET | 1-(5-fluoro-1H-indol-3-yl)butan-2-amine | 1380137-98-3 |
 | 5-Methyl-αET | 1-(5-methyl-1H-indol-3-yl)butan-2-amine | 1380148-21-9 |
 | 5-MeO-αET | 1-(5-methoxy-1H-indol-3-yl)butan-2-amine | 4765-10-0 |
 | 7-Methyl-αET | 1-(7-methyl-1H-indol-3-yl)butan-2-amine | 13712-80-6 |
 | N-Methyl-5-MeO-αMT (α,N,O-TMS/α,N,O-trimethyl-5-HT) | [1-(5-methoxy-1H-indol-3-yl)propan-2-yl](methyl)amine | 4822-13-3 |
 | Indolylpropylaminopentane (α,N-DPT) | 1-(1H-indol-3-yl)-N-propylpentan-2-amine | |
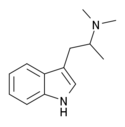 | N,N-Dimethyl-αMT (α,N,N-TMT) | (2-(1H-Indol-3-yl)-1-methyl-ethyl)dimethylamine | 4761-32-4 |
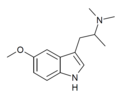 | N,N-Dimethyl-5-MeO-αMT (5-MeO-α,N,N-TMT) | (2-(5-methoxy-1H-Indol-3-yl)-1-methyl-ethyl)dimethylamine | 101831-90-7 |
 | αMDiPT | (2-(1H-Indol-3-yl)-1-methyl-ethyl)diisopropylamine | |
 | MPMI[28] | 3-[(1-methylpyrrolidin-2-yl)methyl]-1H-indole | 143321-54-4 |
 | Lucigenol | (R)-3-(N-methylpyrrolidin-2-ylmethyl)-4-hydoxyindole | 250672-65-2 |
 | 5-MeO-MPMI | 5-Methoxy-3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-1H-indole | 143321-57-7 |
 | 5F-MPMI[29] | (R)-5-fluoro-3-[(1-methylpyrrolidin-2-yl)methyl]-1H-indole | |
 | 5-Br-MPMI | 5-bromo-3-[(1-methylpyrrolidin-2-yl)methyl]-1H-indole | 143322-57-0 |
 | Eletriptan | 3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-5-[2-(benzenesulfonyl)ethyl]-1H-indole | 143322-58-1 |
 | Z5247692566[4][30] | 4-[(3,3-dimethyloxolan-2-yl)methyl]-3-[(1H-indol-3-yl)methyl]morpholine | |
 | BK-NM-AMT (α,N-dimethyl-β-ketotryptamine)[31][32][33] | 1-(1H-indol-3-yl)-2-(methylamino)propan-1-one | |
 | BK-5F-NM-AMT (5-fluoro-α,N-dimethyl-β-ketotryptamine)[34][35][36][33] | 1-(5-fluoro-1H-indol-3-yl)-2-(methylamino)propan-1-one | |
| BK-5Cl-NM-AMT (5-chloro-α,N-dimethyl-β-ketotryptamine)[36][37][38] | 1-(5-chloro-1H-indol-3-yl)-2-(methylamino)propan-1-one | ||
| BK-5Br-NM-AMT (5-chloro-α,N-dimethyl-β-ketotryptamine)[36][39][40] | 1-(5-bromo-1H-indol-3-yl)-2-(methylamino)propan-1-one |
List of substituted β-ketotryptamines
A number of β-ketotryptamines (beta-ketotryptamines) are known.[31][33][36] These compounds are α-alkyl-β-ketotryptamines and are analogous to the cathinones (β-ketoamphetamines) of the related phenethylamine family. Known β-ketotryptamines include BK-NM-AMT, BK-5F-NM-AMT, BK-5Cl-NM-AMT, and BK-5Br-NM-AMT.[31][33][36] They act as monoamine releasing agents.[31][33][36]
Related compounds
A number of related compounds are known, with a similar structure but having the indole core flipped (isotryptamines) and/or replaced with related cores such as indene, indoline, indazole, indolizine, benzothiophene, or benzofuran. Like tryptamines, these related compounds are primarily active as agonists at the 5-HT2 family of serotonin receptors, with applications in the treatment of glaucoma, cluster headaches, or as anorectics.
| Structure | Common name | Chemical name | CAS number |
|---|---|---|---|
| C-DMT [41] | 2-(3H-inden-1-yl)-N,N-dimethylethanamine | ||
 | Dimemebfe | 2-(5-Methoxy-1-benzofuran-3-yl)-N,N-dimethylethanamine | 140853-58-3 |
 | 5-MeO-DiBF | N-[2-(5-Methoxy-1-benzofuran-3-yl)ethyl]-N-(propan-2-yl)propan-2-amine | |
 | 3-APB | 3-(2-aminopropyl)benzofuran | 105909-13-5 |
 | 3-APBT | 1-(1-benzothiophen-3-yl)propan-2-amine | 1201-27-0 |
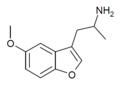 | Mebfap | 3-(2-aminopropyl)-5-methoxybenzofuran | 140853-59-4 |
 | Isotryptamine | 2-indol-1-ylethanamine | 13708-58-2 |
 | isoAMT | 1-indol-1-ylpropan-2-amine | 1227465-67-9 |
| (S)-5,6-Difluoro-isoAMT [42] | (S)-1-(5,6-difluoroindol-1-yl)propan-2-amine | ||
 | Ro60-0175 | (S)-(6-chloro-5-fluoro-1H-indol-1-yl)propan-2-amine | 169675-09-6 |
 | isoDMT | 2-indol-1-yl-N,N-dimethylethanamine | 87482-09-5 |
 | 5-MeO-isoDMT | 2-(5-methoxyindol-1-yl)-N,N-dimethylethanamine | |
 | 6-MeO-isoDMT | 2-(6-methoxyindol-1-yl)-N,N-dimethylethanamine | |
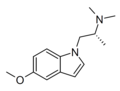 | AAZ-A-154 (DLX-001) | (2R)-1-(5-methoxy-1H-indol-1-yl)-N,N-dimethylpropan-2-amine | 2227169-50-4 |
 | 2ZEDMA | 2-indolizin-1-yl-N,N-dimethylethanamine | |
 | 1ZP2MA | 1-indolizin-1-yl-N-methylpropan-2-amine | |
 | 1Z2MAP1O | 1-indolizin-3-yl-2-(methylamino)propan-1-one | 2110204-31-2 |
 | Example 16 [43][44] | 1-(7-methoxyimidazo[1,5-a]pyridin-3-yl)-N,N-dimethylpropan-2-amine | |
 | Example 1 [45] | 1-(3-methyl-8,9-dihydropyrano[2,3-g]indol-1(7H)-yl)propan-2-amine | |
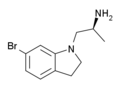 | VER-3323 | (2S)-1-(6-bromo-2,3-dihydroindol-1-yl)propan-2-amine | 259857-99-3 |
 | AL-34662 | 1-((S)-2-Aminopropyl)-1H-indazol-6-ol | 210580-75-9 |
 | O-methyl-AL-34662 | 1-((S)-6-methoxy-2-aminopropyl)-1H-indazole | 210580-60-2 |
 | 7-methyl-AL-34662 | 1-((S)-2-Aminopropyl)-7-methyl-1H-indazol-6-ol | 874668-67-4 |
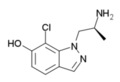 | 7-chloro-AL-34662 | 1-((S)-2-Aminopropyl)-7-chloro-1H-indazol-6-ol | 874881-86-4 |
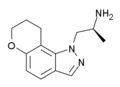 | AL-38022A | (S)-2-(8,9-dihydro-7H-pyrano[2,3-g]indazol-1-yl)-1-methylethylamine | 478132-11-5 |
 | Example 9 [46] | (S)-α-methyl-pyrano[2,3-g]indazole-1(7H)-ethanamine | 478132-12-6 |
 | Example 3 [47] | (S)-7,8-dihydro-α-methyl-1H-[1,4]dioxino[2,3-g]indazole-1-ethanamine | 890087-75-9 |
 | Example 1 [48] | (S)-8,9-dihydro-α,9-dimethylpyrazolo[3,4-f][1,4]benzoxazine-1(7H)-ethanamine | 1373917-69-1 |
 | YM-348 | (2S)-1-(7-ethyl-1H-furo[2,3-g]indazol-1-yl)propan-2-amine | 372163-84-3 |
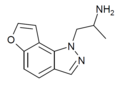 | 2-desethyl-YM-348 [49] | (2S)-1-(1H-furo[2,3-g]indazol-1-yl)propan-2-amine | 748116-94-1 |
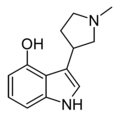 | I-32 [50] | 3-(1-methylpyrrolidin-3-yl)-1H-indol-4-ol | |
 | 2-Azapsilocin (Psilocin indazole analogue, P-6)[51] | 3-[2-(dimethylamino)ethyl]-1H-indazol-4-ol | |
 | 4-Aza-5-MeO-DPT (P-11) | N-[2-(5-methoxy-1H-pyrrolo[3,2-b]pyridin-3-yl)ethyl]-N-propylpropan-1-amine | |
 | 5-Aza-4-MeO-DiPT (P-36) | N-[2-(4-methoxy-1H-pyrrolo[3,2-c]pyridin-3-yl)ethyl]-N-(propan-2-yl)propan-2-amine | |
 | 7-Aza-5-MeO-DiPT (P-19) | N-[2-(5-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)ethyl]-N-(propan-2-yl)propan-2-amine | |
 | VU6067416[52] | 3-(1,2,5,6-tetrahydropyridin-3-yl)-5-bromo-1H-indazole | |
 | (R)-69 | 3-[(5R)-5-methyl-1,2,5,6-tetrahydropyridin-3-yl]-1H-pyrrolo[2,3-b]pyridine | |
 | SN-22 | 3-(1-methylpiperidin-4-yl)-1H-indole | 17403-07-5 |
Tryptamine-based therapeutics
| Drug | Mechanism | Treatment | Effect | Structure |
|---|---|---|---|---|
| Sumatriptan[53] | 5-HT1B and 5-HT1D agonist | Migraine Headaches | Vasoconstriction of brain blood vessels |  |
| Rizatriptan[53] | 5-HT1B and 5-HT1D agonist | Migraine Headaches | Vasoconstriction of brain blood vessels |  |
| Zolmitriptan[53] | 5-HT1B and 5-HT1D agonist | Migraine Headaches | Vasoconstriction of brain blood vessels |  |
| Almotriptan[53] | 5-HT1B and 5-HT1D agonist | Migraine Headaches | Vasoconstriction of brain blood vessels |  |
| Eletriptan[53] | 5-HT1B and 5-HT1D agonist | Migraine Headaches | Vasoconstriction of brain blood vessels |  |
| Frovatriptan[53] | 5-HT1B and 5-HT1D agonist | Migraine Headaches | Vasoconstriction of brain blood vessels |  |
| Naratriptan[53] | 5-HT1B and 5-HT1D agonist | Migraine Headaches | Vasoconstriction of brain blood vessels |  |
Further reading
See also
- Ergoline
- Lysergamide
- Iboga alkaloid
- Substituted amphetamine
- Substituted benzofuran
- Substituted cathinone
- Substituted methylenedioxyphenethylamine
- Substituted phenethylamine
- 2C, DOx, 25-NB
- List of miscellaneous 5-HT2A receptor agonists
References
- ^ Chemistry, University of; Prague, Technology. "Concentrations of psychoactive compounds in mushrooms found to be extremely variable". phys.org. Retrieved 2022-12-26.
- ^ Toro-Sazo M, Brea J, Loza MI, Cimadevila M, Cassels BK (2019). "5-HT2 receptor binding, functional activity and selectivity in N-benzyltryptamines". PLOS ONE. 14 (1): e0209804. Bibcode:2019PLoSO..1409804T. doi:10.1371/journal.pone.0209804. PMC 6328172. PMID 30629611.
- ^ Jensen N. Tryptamines as Ligands and Modulators of the Serotonin 5-HT2A Receptor and the Isolation of Aeruginascin from the Hallucinogenic Mushroom Inocybe aeruginascens. PhD thesis, University of Göttingen, 2004
- ^ a b Lyu J, Kapolka N, Gumpper R, Alon A, Wang L, Jain MK, et al. (December 2023). "AlphaFold2 structures template ligand discovery". bioRxiv. doi:10.1101/2023.12.20.572662. PMC 10769324. PMID 38187536.
- ^ McKay JB, Parkhurst RM, Silverstein RM, Skinner WA (October 1963). "Analogues of Psilocin and Lysergic acid diethylamide I. Chloro, Nitro, and Amino Derivatives of 3-Substituted Indoles". Canadian Journal of Chemistry. 41 (10): 2585–2590. doi:10.1139/v63-378.
- ^ Klein AK, Chatha M, Laskowski LJ, Anderson EI, Brandt SD, Chapman SJ, McCorvy JD, Halberstadt AL (April 2021). "Investigation of the Structure-Activity Relationships of Psilocybin Analogues". ACS Pharmacology & Translational Science. 4 (2): 533–542. doi:10.1021/acsptsci.0c00176. PMC 8033608. PMID 33860183.
- ^ Pham DN, Chadeayne AR, Golen JA, Manke DR (February 2021). "Psilacetin derivatives: fumarate salts of the meth-yl-ethyl, meth-yl-allyl and diallyl variants of the psilocin prodrug". Acta Crystallographica Section E. 77 (Pt 2): 101–106. Bibcode:2021AcCrE..77..101P. doi:10.1107/S2056989021000116. PMC 7869532. PMID 33614134.
- ^ Sherwood AM, Burkhartzmeyer EK, Williamson SE, Baumann MH, Glatfelter GC. Psychedelic-like Activity of Norpsilocin Analogues. ACS Chem Neurosci. 2024 Jan 17;15(2):315-327. doi:10.1021/acschemneuro.3c00610 PMID 38189238
- ^ WO 2023115167, Banister S, Jorgensen W, Jinlong T, "Compounds", published 29 June 2023, assigned to Psylo Pty Ltd.
- ^ WO 2021179091, Kozikowski A, Shaprio G, Tueckmantel W, McCorvy J, "3-(2-(Aminoethyl)-indol-4-ol derivatives, methods of preparation thereof, and the use as 5-HT2 receptor modulators", published 16 September 2021, assigned to Bright Minds Biosciences Inc. and The Medical College Of Wisconsin Inc.
- ^ WO 2021101926, Stamets PE, "Tryptamine Compositions for Enhancing Neurite Outgrowth.", published 2021-05-27, assigned to Stamets Paul Edward.
- ^ WO 2021168082, Kruegel AC, Sporn J, "Specific Tryptamines for use in the Treatment of Mood Disorders.", published 26 August 2021
- ^ Xu YC, Schaus JM, Walker C, Krushinski J, Adham N, Zgombick JM, Liang SX, Kohlman DT, Audia JE (February 1999). "N-Methyl-5-tert-butyltryptamine: A novel, highly potent 5-HT1D receptor agonist". Journal of Medicinal Chemistry. 42 (3): 526–31. doi:10.1021/jm9805945. PMID 9986723.
- ^ WO 2022235927, Kruegel AC, "Novel Tryptamines and Methods of Treating Mood Disorders", published 10 November 2022, assigned to Gilgamesh Pharmaceuticals, Inc.
- ^ Shaw E, Woolley DW (April 1953). "The synthesis of nitro-and aminoindoles analogous to serotonin". Journal of the American Chemical Society. 75 (8): 1877–1881. doi:10.1021/ja01104a029.
- ^ Rabin RA, Regina M, Doat M, Winter JC (May 2002). "5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens". Pharmacology, Biochemistry, and Behavior. 72 (1–2): 29–37. doi:10.1016/s0091-3057(01)00720-1. PMID 11900766. S2CID 6480715.
- ^ Glennon RA, Schubert E, Jacyno JM, Rosecrans JA (November 1980). "Studies on several 7-substituted N,N-dimethyltryptamines". Journal of Medicinal Chemistry. 23 (11): 1222–6. doi:10.1021/jm00185a014. PMID 6779006.
- ^ WO 2023115166, Banister S, Jorgensen W, Jinlong T, "Compounds", published 29 June 2023, assigned to Psylo Pty Ltd.
- ^ a b Ries RK, Miller SC, Fiellin DA (2009). Principles of Addiction Medicine. Lippincott Williams & Wilkins. pp. 216–218. ISBN 978-0-7817-7477-2.
- ^ a b c Laing RR (2003). Hallucinogens: A Forensic Drug Handbook. Academic Press. pp. 102–. ISBN 978-0-12-433951-4.
- ^ a b c d e Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 641–. ISBN 978-1-60913-345-0.
- ^ Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology. 559 (2–3): 132–7. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ^ Blough BE, Landavazo A, Partilla JS, Decker AM, Page KM, Baumann MH, Rothman RB (October 2014). "Alpha-ethyltryptamines as dual dopamine-serotonin releasers". Bioorganic & Medicinal Chemistry Letters. 24 (19): 4754–4758. doi:10.1016/j.bmcl.2014.07.062. PMC 4211607. PMID 25193229.
- ^ Nonaka R, Nagai F, Ogata A, Satoh K (December 2007). "In vitro screening of psychoactive drugs by [(35)S]GTPgammaS binding in rat brain membranes". Biological & Pharmaceutical Bulletin. 30 (12): 2328–33. doi:10.1248/bpb.30.2328. PMID 18057721.
- ^ Feldman JM, Chapman B (December 1975). "Monoamine oxidase inhibitors: nature of their interaction with rabbit pancreatic islets to alter insluin secretion". Diabetologia. 11 (6): 487–94. doi:10.1007/bf01222097. PMID 1107123.
- ^ Huang XM, Johnson MP, Nichols DE (July 1991). "Reduction in brain serotonin markers by alpha-ethyltryptamine (Monase)". European Journal of Pharmacology. 200 (1): 187–90. doi:10.1016/0014-2999(91)90686-K. PMID 1722753.
- ^ Chang-Fong J, Addo J, Dukat M, Smith C, Mitchell NA, Herrick-Davis K, Teitler M, Glennon RA (January 2002). "Evaluation of isotryptamine derivatives at 5-HT(2) serotonin receptors". Bioorganic & Medicinal Chemistry Letters. 12 (2): 155–8. doi:10.1016/s0960-894x(01)00713-2. PMID 11755343.
- ^ US 5607951, Macor JE, Wythes MJ, "Indole derivatives", issued 4 March 1997, assigned to Pfizer, Inc.
- ^ WO 2022256554, Wallach J, Dybek M, "Fluorinated Tryptamine Compounds, Analogues Thereof, and Methods Using Same.", published 8 December 2022, assigned to University Of The Sciences.
- ^ Callaway E (18 January 2024). "AlphaFold found thousands of possible psychedelics. Will its predictions help drug discovery?". Nature News. 626 (7997): 14–15. Bibcode:2024Natur.626...14C. doi:10.1038/d41586-024-00130-8. PMID 38238624. S2CID 267040499.
- ^ a b c d Blough BE, Decker AM, Landavazo A, Namjoshi OA, Partilla JS, Baumann MH, Rothman RB (March 2019). "The dopamine, serotonin and norepinephrine releasing activities of a series of methcathinone analogs in male rat brain synaptosomes". Psychopharmacology (Berl). 236 (3): 915–924. doi:10.1007/s00213-018-5063-9. PMC 6475490. PMID 30341459.
- ^ "1-(1H-indol-3-yl)-2-(methylamino)propan-1-one". PubChem. Retrieved 11 November 2024.
- ^ a b c d e "Specialized combinations for mental disorders or mental enhancement". Google Patents. 7 June 2024. Retrieved 4 November 2024.
- ^ "1-(5-fluoro-1H-indol-3-yl)-2-(methylamino)propan-1-one". PubChem. Retrieved 11 November 2024.
- ^ "β-Oxo-5-fluoro-α-methyl-NMT". Isomer Design. 10 November 2024. Retrieved 11 November 2024.
- ^ a b c d e f "Advantageous tryptamine compositions for mental disorders or enhancement". Google Patents. 20 September 2021. Retrieved 11 November 2024.
- ^ "1-(5-chloro-1H-indol-3-yl)-2-(methylamino)propan-1-one". PubChem. Retrieved 11 November 2024.
- ^ "β-Oxo-5-chloro-α-methyl-NMT". Isomer Design. 10 November 2024. Retrieved 11 November 2024.
- ^ "1-(5-bromo-1H-indol-3-yl)-2-(methylamino)propan-1-one". PubChem. Retrieved 11 November 2024.
- ^ "β-Oxo-5-bromo-α-methyl-NMT". Isomer Design. 10 November 2024. Retrieved 11 November 2024.
- ^ "PiHKAL·info". Isomer Design. 11 November 2024. Retrieved 31 January 2025.
- ^ Chang-Fong J, Addo J, Dukat M, Smith C, Mitchell NA, Herrick-Davis K, Teitler M, Glennon RA (January 2002). "Evaluation of isotryptamine derivatives at 5-HT(2) serotonin receptors". Bioorg Med Chem Lett. 12 (2): 155–158. doi:10.1016/s0960-894x(01)00713-2. PMID 11755343.
Detailed re-examination of a compound previously reported to display 100-fold 5-HT2C selectivity [i.e., S(+)-5,6-difluoro-α-methylisotryptamine] revealed that its selectivity versus 5-HT2A receptors was, at best, only 10-fold.
- ^ Kargbo RB. Neuropharmacological Advances: Harnessing 5-HT2A Receptor Modulators and Psychoplastogens. ACS Med Chem Lett. 2024 Jan 23;15(2):171-173. doi:10.1021/acsmedchemlett.4c00003 PMID 38352827
- ^ Powell NA, Chytil M. Imidazopyridine psychoplastogens and uses thereof. WO 2023/114844
- ^ US granted 7012090, Chen HH, May JA, "Pyranoindoles for treating glaucoma", published 17 March 2000, issued 14 March 2006, assigned to Alcon, Inc.
- ^ US granted 6881749, Chen HH, May JA, Severns BS, "Pyranoindazoles and their use for the treatment of glaucoma", published 3 June 2004, issued 19 April 2005, assigned to Alcon, Inc.
- ^ US granted 7425572, Chen HH, May JA, "Use of dioxindoindazoles and dioxoloindazoles for treating glaucoma", published 8 June 2006, issued 16 September 2008, assigned to Alcon, Inc.
- ^ US granted 7268131, Dantanarayana AP, May JA, "Substituted [1,4]oxazino[2,3-g]indazoles for the treatment of glaucoma", published 15 December 2005, issued 11 September 2007, assigned to Alcon, Inc.
- ^ Shimada I, Maeno K, Kazuta K, Kubota H, Kimizuka T, Kimura Y, et al. (February 2008). "Synthesis and structure-activity relationships of a series of substituted 2-(1H-furo[2,3-g]indazol-1-yl)ethylamine derivatives as 5-HT2C receptor agonists". Bioorganic & Medicinal Chemistry. 16 (4): 1966–82. doi:10.1016/j.bmc.2007.10.100. PMID 18035544.
- ^ WO 2022120475, Slassi A, Araujo J, Higgins G, "3-Cyclic Amine-Indole Derivatives as Serotonergic Agents for the Treatment of CNS Disorders.", published 16 June 2022, assigned to Mindset Pharma Inc.
- ^ WO 2023115165, Banister S, Jorgensen W, Jinlong T, "Compounds", published 29 June 2023, assigned to Psylo Pty Ltd.
- ^ Jayakodiarachchi N, et al. Evaluation of the Indazole Analogs of 5-MeO-DMT and Related Tryptamines as Serotonin Receptor 2 Agonists. ACS Med. Chem. Lett. 2024 doi:10.1021/acsmedchemlett.3c00566
- ^ a b c d e f g "Serotonin Receptor Agonists (Triptans)", LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2012, PMID 31644023, retrieved 2020-10-15